Glassy fast ionic conductors can be used as solid electrolytes, cathode materials, conducting fibers and electrochromic glasses due to their high ionic conductivities and good transparency. While the conductivity of the conductors is highly dependent on the organization of glass networks, it is very difficult to finely characterize the glass structure, and thus the relation between the conductivity and glass structures was rarely reported. Solid-state nuclear magnetic resonance (NMR) is extremely suitable for probing glass structures due to its flexible and comprehensive capabilities in detecting the structure information of vitreous materials at the atomic scale.
Currently, the doping of transition metal ions in phosphate glasses has attracted wide attention since the transition metal ions can significantly modify electrical conductivity and optical properties of glasses. The addition of alkali halide might further promote the mobility of ions.
Most recently, a research team led by Shanghai Institute of Optics and Fine Mechanics, Chinese Academy of Sciences, has investigated the structures of MoO3-doped phosphate ionic conducting glasses in detail using multiple solid-state NMR technologies. The study was published in The Journal of Physical Chemistry C.
In their experiments, the PO5/2 was substituted by the same amount of MoO3. The local environments of 31P, 7Li, and 35Cl were characterized by single-pulse NMR spectra. The distribution and connectivity of phosphorus networks were studied by two-dimensional NMR experiments. Raman spectra were employed to detect the local environments of Mo. The complex impedances have also been measured to obtain the ionic conductivities of these glasses.
They found that the ionic conductivity was enhanced about 250 times with the substitution of PO5/2 by MoO3, and the maximum value reached 1.05×10-5 S·cm-1 at 18 °C for x = 70. In these glasses, Cl- ions only bonded to Li+ ions but not P5+ or Mo6+. Within the range of x ≤ 20, phosphorus phases dominated the glasses networks and phosphorus chains were broken into dimer phosphorus species Q(1)0Mo by Li+ ions. The average number of Li+ ions in the phosphorus phase was highly increased. The increase of ionic conductivity should be mainly due to the increase of Li+ ion concentration in the phosphorus phases and the generation of looser dimer structure.
However, within the range of 20 ≤ x ≤ 70, the dimer phosphorus species were gradually broken into orthophosphate Q(0)1Mo species dispersed in molybdenum phases. Since the decrease of PO5/2, a large number of Li+ ions gradually transferred from phosphorus oxide phases into molybdenum oxide phases. In this range, the increase of ionic conductivity should be due to the increase of Li+ ion conductivity in molybdenum phases.
This study develops a comprehensive glass structure evolution model with compositions and presents a profound insight into the effects of compositions and structures on ion conductivity. The results of this work could be valuable for composition designs of glass electrolytes.
This work was supported by the National Natural Science Foundation of China.
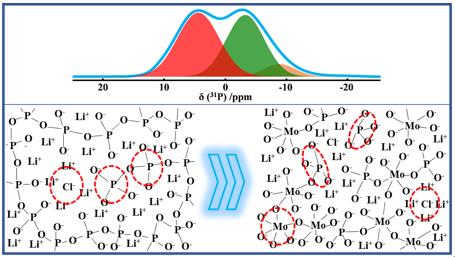
The structure evolution of fast ionic conducting glasses characterized using solid-state NMR technologies.
Article website:
https://pubs.acs.org/doi/10.1021/acs.jpcc.0c00171
Contact:
Mr. CAO Yong
General Administrative Office
Shanghai Institute of Optics and Fine Mechanics, CAS
Email: caoyong@siom.ac.cn