Free of some degree of crystallinity, single-component Al2O3 atomic-level glass cannot be obtained by conventional melt quenching methodology. This is because extremely high quenching rates are needed to achieve an amorphous state for glass intermediates or modifiers oxide such as alumina whose calculated rate is as high as 107Ks-1. So far, glassy alumina was obtained only from equilibrium conditions, which limits their dimensions to thin films or nanoparticles (<5 μm). In last decade, the atomic structure of glassy Al2O3 has been revealed via solid-state NMR based on those low dimensional glassy aluminas.
In 2007 Prof. ZHANG Long from Shanghai Institute of Optics and Fine Mechanics, Chinese Academy of Sciences, reported an aqueous Al-lactate sol-gel route to amorphous alumina in a conference paper, which was recently proven to be glassy on the atomistic level. Because of the central importance of this finding, they also investigated in detail the formation of this alumina glass from the sol to gel to glass transitions. This, to the best of our knowledge, is the first time that a real bulk-scale alumina glass has been obtained. Their findings were published in Acta Materialia.
In their experiment, detailed Al NMR analysis, supported by several other analytical methods, indicated the formation of a metal coordination network based on aluminum cations-lactate ligand interactions, which occurred during the sol-gel transition. This water soluble metal coordination network demonstrated a facile reversible sol-gel-sol transition based on concentration-driven formation and breaking of the intramolecular non-covalent interactions between the monomers.
Subsequent annealing of the xerogel by gradual heating to 500–700°C promoted the formation of the interconnecting Al–O–Al bonds, concomitant with the removal of the lactate ligands upon heating, resulting in pure alumina glass as the final product. A glass transition temperature (Tg) of Al2O3 glass was observed at ~670°C for the first time.
Similar observations were reported here also for aluminum citrate, which indicated that the Al-chelating approach to alumina glass was general.
The first experimental evidence of triply coordinated oxygen O in the covalent oxide glasses was directly observed via O solid-state NMR in this sol-gel derived Al2O3 glass as well.
Therefore, the atomistic origin of low glass forming ability of Al2O3 was revealed to the triply shared oxygen atoms, which violates Zachariasens's rule of glass formation since they reduce the flexibility of Al–O–Al bond angles and prefer denser, edge shared polyhedral structures that are prone to crystallization.

Fig. 1. (a) Flowchart depicting the sol-gel route of Al2O3 glass. (b) Photos of representative samples in the gel-glass transition process based on aluminum-lactate route. (Image by SIOM)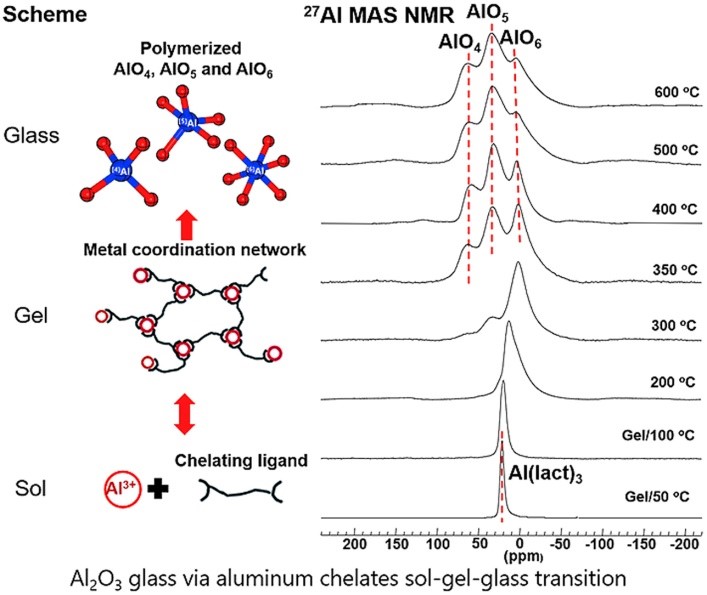
Fig. 2. (Left) The scheme of sol-gel-glass transition; (Right) 130.3 MHz solid-state Al NMR for gel-glass transition. (Image by SIOM)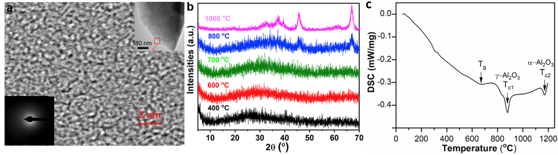
Fig. 3. (a) Gel-derived Al2O3 glass HRTEM image of the selected thin-edge area (b) XRD patterns of gel derived Al2O3 sequentially annealed. (Image by SIOM)
Article website: https://doi.org/10.1016/j.actamat.2019.05.062
Contact:
Mr. Cao Yong
General Administrative Office
Shanghai Institute of Optics and Fine Mechanics, CAS
Email: caoyong@siom.ac.cn